The photoelectric effect
Gennaio 27th, 2023 | by Marcello Colozzo |
Photoelectric effect. Introduction
In Planck's hypothesis (1900) the quantization of the oscillator system is a schematization of the matter in equilibrium with the radiation. In 1905, Albert Einstein reinterpreted the aforementioned hypothesis by introducing the notion ofphoton (or quanta of light). More specifically, Einstein elaborated a theoretical model of the photoelectric effect. Towards the end of the 19th century, Hertz, Hallfacks and others had observed that by irradiating a metal surface in a high vacuum, with a sufficiently high frequency radiation, there was an emission of electrons.In particular, the metal surface is a photocathode of an electron tube, so the electrons emitted by the photocathode reach the anode thanks to a assigned potential difference V which can be changed via a potentiometer. The resulting current intensity I is then measured by an ammeter. Experimentally, a "reverse field" is observed

and a saturation value:

Other notable experimental facts:
- Electron emission occurs only if ν > ν0, where ν is the frequency of the incident radiation and ν0 a metal-dependent characteristic value.
- The speed v of the emitted electrons does not exceed a value vmax and the latter increases linearly with the frequency of the incident radiation. The aforesaid maximum speed is instead independent of the intensity of the radiation.
- The flux of emitted electrons (number of electrons emitted per unit area and per unit time) depends on the intensity of the radiation.
The listed experimental facts cannot be explained by classical electrodynamics. For example, by illuminating a large metal surface with very low intensity radiation, the emitted electronic flux is equally low (point 3). However, the emission process is instantaneous, while according to classical electrodynamics, the electron acquires sufficient energy to be torn from the metal after a relatively long time.
What did Einstein do?
Contrary to Planck, according to whom electromagnetic radiation is quantized only in the processes of emission/absorption, Einstein extended the aforementioned quantization to the processes of propagation. It would follow from this that there are elements of elements of energy ε=hν which he called quanta of light or photons.
In this revolutionary conceptual framework, the photoelectric effect is a collision process between the incident photons and the atomic electrons of the metal. Precisely, let ε=hν the energy of the photon hitting an atom of the metal. An electron of such an atom can be emitted only if ε=hν > L0 where L0 is the mechanical work necessary to strip the electron from the atom with the consequent emission from the metal. Let's put:

where ν0 is a characteristic frequency of the metal (see point 1). It follows that if ε > hν0 and therefore if ν > ν0, the electron leaves the metal with energy

for which the maximum energy of the emitted electron is

which corresponds to a maximum speed vmax which depends linearly on the frequency of the radiation (point 2). Note that the maximum speed does not depend on the intensity of the radiation but only on the frequency. This explains points 2 and 3.
An experimental verification of the above equation was performed by Millikan. The photon hypothesis gives light a dual nature (corpuscular and wave). However, the corpuscular aspect is different from that proposed by Newton, according to whom the corpuscles of light were material points endowed with a given kinetic energy. On the contrary, starting from the fact that an electromagnetic field, in addition to carrying an energy E, carries a momentum E/c, Einstein assigned to the single photon a momentum

Then recalling the relativistic expression of the kinetic energy of a particle of mass at rest m:

it follows that the rest mass of the photons is zero.


 Congettura di Riemann
Congettura di Riemann Trasformata discreta di Fourier
Trasformata discreta di Fourier
 Trasformata di Fourier nel senso delle distribuzioni
Trasformata di Fourier nel senso delle distribuzioni Trasformata di Fourier
Trasformata di Fourier  Infinitesimi ed infiniti
Infinitesimi ed infiniti Limiti notevoli
Limiti notevoli Punti di discontinuità
Punti di discontinuità Misura di Peano Jordan
Misura di Peano Jordan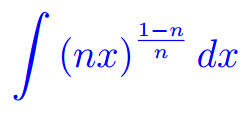 Eserciziario sugli integrali
Eserciziario sugli integrali Differenziabilità
Differenziabilità  Differenziabilità (2)
Differenziabilità (2) Esercizi sui limiti
Esercizi sui limiti Appunti sulle derivate
Appunti sulle derivate Studio della funzione
Studio della funzione Esercizi sugli integrali indefiniti
Esercizi sugli integrali indefiniti Algebra lineare
Algebra lineare Analisi Matematica 2
Analisi Matematica 2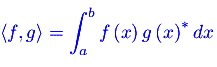 Analisi funzionale
Analisi funzionale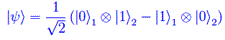 Entanglement quantistico
Entanglement quantistico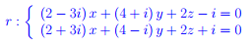 Spazio complesso
Spazio complesso Biliardo di Novikov
Biliardo di Novikov Intro alla Meccanica quantistica
Intro alla Meccanica quantistica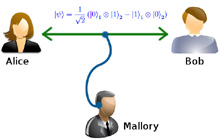 Entanglement Quantistico
Entanglement Quantistico